- Home Page
-
Solutions
-
CRO Services
- 06 Integrated Solutions
-
11 Featured Solutions
- 01 Innovative Antibody Discovery Platforms With the Super-trillion Ab Libraries
- 02 Screening With the Sub-library of Super-trillion Fully Human Ab Library
- 03 Screening With the Sub-library of Super-trillion Single Domain Ab Library
- 04 Screening With the Sub-library of Super-trillion Common Light Chain Ab Library
- 05 MIT Mouse Recombinant mAb Customization
- 06 MIT Alpaca Recombinant mAb Customization
- 07 Monoclonal and Polyclonal Anti-drug Antibody Customization
- 08 High-quality Gram-level Antibody Production
- 09 Rapid Protein Expression and Purification Service
- 10 Antigen and Antibody Structure Analysis
- 11 Antibody Specificity Analysis
-
11 Staged Solutions
- 01 In-depth Antibody Humanization
- 02 Ultimate Antibody Affinity Maturation
- 03 In Vitro Efficacy Evaluation
- 04 In Vivo Efficacy Evaluation
- 05 Antibody Pharmacokinetic Analysis
- 06 Multidimensional Characterization for Protein
- 07 Antibody Production Cell Line Development
- 08 Research Overexpression Cell Line Construction
- 09 Fermentation Process Development
- 10 Purification Process Development
- 11 Formulation Development
- CPO Projects
-
CRO Services
- Products
- Media
- Activities
- About Us
- Careers
- Supports
-
06 Integrated Solutions
-
11 Featured Solutions
-
11 Staged Solutions
Service Overviews
Background: Pharmacokinetic (PK) studies are required for evaluating new molecular entities and is an essential prerequisite for interpreting preclinical efficacy and toxicology results.
Methods: Pharmacokinetic (PK) studies involve administration of drugs through different routes and samples collection. The plasma-drug concentration at different time points can be measured by ELISA and analyzed for PK data by WinNonlin.
Advantages: With AAALAC accredited animal room facilities, Sanyou Bio provides you with PK assay development and validation in various species, as well as one-stop services for the high-quality antigen/antibody production.
Cases: Sanyou Bio has accumulated rich experience in PK studies, including TNFR2, CD40 and VEGF, etc.
Service Overviews
Background: Pharmacokinetic (PK) studies are required for evaluating new molecular entities and is an essential prerequisite for interpreting preclinical efficacy and toxicology results.
Methods: Pharmacokinetic (PK) studies involve administration of drugs through different routes and samples collection. The plasma-drug concentration at different time points can be measured by ELISA and analyzed for PK data by WinNonlin.
Advantages: With AAALAC accredited animal room facilities, Sanyou Bio provides you with PK assay development and validation in various species, as well as one-stop services for the high-quality antigen/antibody production.
Cases: Sanyou Bio has accumulated rich experience in PK studies, including TNFR2, CD40 and VEGF, etc.
Service Contents
Service
Service Details
Client Provides
Delivery
Time
PK study
1. Varied routes of administration
2. Alternative species
3. PK assay development and validation
4. PK analysis
Antigen/Antibody/ADC
Report
8–10 weeks
Anti-drug antibody detection
1. Serum collection
2. Anti-drug antibody detection
Antigen/Antibody/ADC
Report
Antibody production
1. Plasmid construction
2. Expression and purification
3. Quality control
Sequence
Report
Service Highlights
1. High precision PK solution
Four molecular types, five animal species, six drug delivery routes and seven types of biological substrates are available for selection, and high-precision PK solutions can be customized according to molecular characteristics.
2. Standardized Laboratory Animal Facility
Sanyou Bio has rented SPF/Elite level animal facility, high-quality laboratory mice and meet animal ethics requirements.
3. One-stop high-quality customized service
One-stop services from the high-quality antigen/antibody to PK assay development and validation .
4. Fast and high-quality delivery
We utilize effective method development, transfer, set-up, and validation techniques to identify and quantitate drug in a timely and cost-effective method.
Service Features
1. Diverse molecular types
Monoclonal antibody, multi-specific antibody, recombinant protein, ADC drugs, etc.
2. Complete animal species
Mouserat, rabbit, dog, minipig, model animal, etc.
3. Various routes of administration
Intravenous, oral, subcutaneous, abdominal, muscle, local administration, etc.
4. Comprehensive biological matrix
Whole blood, plasma, serum, urine and feces, bile, cerebrospinal fluid, various tissues, etc.
5. Standardized Laboratory Animal Facility
Sanyou Bio has rented SPF/Elite animal facility, high-quality laboratory mice and meet animal ethics requirements.
6. Advanced facilities and equipment
Case Stastics
1. Pharmacokinetics of Monoclonal antibody
Objective: To evaluate the PK characteristics of mAb A in rats.
Methods: The mAb were intravenously administered to SD rats through the tail vein, and the serum concentration at different time points were measured by ELISA.
Results: Fig. 1 represents the serum concentration-time curve of each rat after the administration of mAb A, The parameters fitted by WinNonlin are shown in Table 1.
Conclusions: The results showed that the half-life T1/2, AUC (0-t), and Cmax of mAb molecule A are (34.14±1.67) h, (12358.76±1424.08) h·μg/mL, and (315.17±47.95) μg/mL, respectively.
Fig. 1 Serum concentration-time curves of mAb A
Table 1 Parameters curves of mAb A
Group
Animal ID
T1/2
(h)
Tmax
(h)
Cmax
(μg/ml)
AUC 0-t
(h*μg/ml)
AUC 0-∞
(h*μg/ml)
Vd
(ml/kg)
CL
(ml/h/kg)
MRT0-∞(h)
G1 A i.v.
1
33.56
0.5
285.69
13835.65
13853.76
161.81
1.08
122.32
2
32.84
0.5
370.50
10994.13
11004.53
201.53
1.36
141.76
3
36.02
0.5
289.31
12246.52
12257.52
182.38
1.22
107.45
mean
34.14
0.50
315.17
12358.76
12371.94
181.91
1.22
123.84
SD
1.67
0.00
47.95
1424.08
1428.06
19.86
0.14
17.21
2. Pharmacokinetics of Bispecific antibody
Objectives: To evaluate the PK characteristics of bispecific antibody B in rats.
Methods: The bispecific antibody molecules were intravenously administered to SD rats through the tail vein, and the serum concentration at different time points was measured by ELISA.
Results: Figure 2 represents the serum concentration-time curve of each rat after the administration of B, and the parameters fitted by WinNonlin are shown in Table 2.
Conclusions: The results showed that the half-life T1/2, AUC (0-t), and Cmax of bispecific antibody B are (153.11± 6.49) h, (2073.29 ± 80.01) h·μg/mL, and (35.68 ± 4.54) μg/mL, respectively.
Fig. 2 serum concentration-time curves of BsAb B
Table 2 parameters curves of BsAb B
Group
Animal ID
T1/2
(h)
Cmax
(μg/ml)
Tmax
(h)
AUC 0-t
(h*μg/ml)
AUC 0-∞
(h*μg/ml)
CL
(ml/h/kg)
MRT0-∞
(h)
G1 B i.v.
1
148.07
33.57
0.50
2165.66
2194.60
2.06
161.45
2
160.43
32.58
0.50
2028.51
2060.49
2.19
167.71
3
150.83
40.90
0.50
2025.70
2053.78
2.01
128.81
mean
153.11
35.68
0.50
2073.29
2102.96
2.09
152.66
SD
6.49
4.54
0.00
80.01
79.44
0.10
20.89
Service Contents
|
Service |
Service Details |
Client Provides |
Delivery |
Time |
|
PK study |
1. Varied routes of administration 2. Alternative species 3. PK assay development and validation 4. PK analysis |
Antigen/Antibody/ADC |
Report |
8–10 weeks |
|
Anti-drug antibody detection |
1. Serum collection 2. Anti-drug antibody detection |
Antigen/Antibody/ADC |
Report |
|
|
Antibody production |
1. Plasmid construction 2. Expression and purification 3. Quality control |
Sequence |
Report |
Service Highlights
-
1. High precision PK solution
-
Four molecular types, five animal species, six drug delivery routes and seven types of biological substrates are available for selection, and high-precision PK solutions can be customized according to molecular characteristics.
-
-
2. Standardized Laboratory Animal Facility
-
Sanyou Bio has rented SPF/Elite level animal facility, high-quality laboratory mice and meet animal ethics requirements.
-
-
3. One-stop high-quality customized service
-
One-stop services from the high-quality antigen/antibody to PK assay development and validation .
-
-
4. Fast and high-quality delivery
-
We utilize effective method development, transfer, set-up, and validation techniques to identify and quantitate drug in a timely and cost-effective method.
-
Service Features
-
1. Diverse molecular types
-
Monoclonal antibody, multi-specific antibody, recombinant protein, ADC drugs, etc.
-
-
2. Complete animal species
-
Mouserat, rabbit, dog, minipig, model animal, etc.
-
-
3. Various routes of administration
-
Intravenous, oral, subcutaneous, abdominal, muscle, local administration, etc.
-
-
4. Comprehensive biological matrix
-
Whole blood, plasma, serum, urine and feces, bile, cerebrospinal fluid, various tissues, etc.
-
-
5. Standardized Laboratory Animal Facility
-
Sanyou Bio has rented SPF/Elite animal facility, high-quality laboratory mice and meet animal ethics requirements.
-
- 6. Advanced facilities and equipment
Case Studies
1. Pharmacokinetics of Monoclonal antibody
Objective: To evaluate the PK characteristics of mAb A in rats.
Methods: The mAb were intravenously administered to SD rats through the tail vein, and the serum concentration at different time points were measured by ELISA.
Results: Fig. 1 represents the serum concentration-time curve of each rat after the administration of mAb A, The parameters fitted by WinNonlin are shown in Table 1.
Conclusions: The results showed that the half-life T1/2, AUC (0-t), and Cmax of mAb molecule A are (34.14±1.67) h, (12358.76±1424.08) h·μg/mL, and (315.17±47.95) μg/mL, respectively.
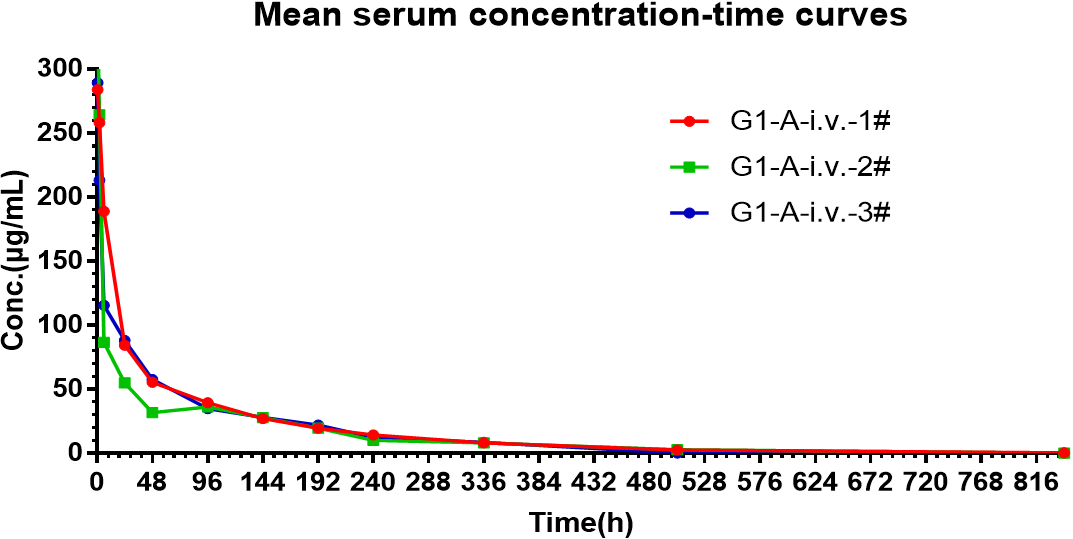
Fig. 1 Serum concentration-time curves of mAb A
Table 1 Parameters curves of mAb A
|
Group
|
Animal ID
|
T1/2
(h)
|
Tmax
(h)
|
Cmax
(μg/ml)
|
AUC 0-t
(h*μg/ml)
|
AUC 0-∞
(h*μg/ml)
|
Vd
(ml/kg)
|
CL
(ml/h/kg)
|
MRT0-∞(h)
|
|
G1 A i.v.
|
1
|
33.56
|
0.5
|
285.69
|
13835.65
|
13853.76
|
161.81
|
1.08
|
122.32
|
|
2
|
32.84
|
0.5
|
370.50
|
10994.13
|
11004.53
|
201.53
|
1.36
|
141.76
|
|
|
3
|
36.02
|
0.5
|
289.31
|
12246.52
|
12257.52
|
182.38
|
1.22
|
107.45
|
|
|
mean
|
34.14
|
0.50
|
315.17
|
12358.76
|
12371.94
|
181.91
|
1.22
|
123.84
|
|
|
SD
|
1.67
|
0.00
|
47.95
|
1424.08
|
1428.06
|
19.86
|
0.14
|
17.21
|
2. Pharmacokinetics of Bispecific antibody
Objectives: To evaluate the PK characteristics of bispecific antibody B in rats.
Methods: The bispecific antibody molecules were intravenously administered to SD rats through the tail vein, and the serum concentration at different time points was measured by ELISA.
Results: Figure 2 represents the serum concentration-time curve of each rat after the administration of B, and the parameters fitted by WinNonlin are shown in Table 2.
Conclusions: The results showed that the half-life T1/2, AUC (0-t), and Cmax of bispecific antibody B are (153.11± 6.49) h, (2073.29 ± 80.01) h·μg/mL, and (35.68 ± 4.54) μg/mL, respectively.
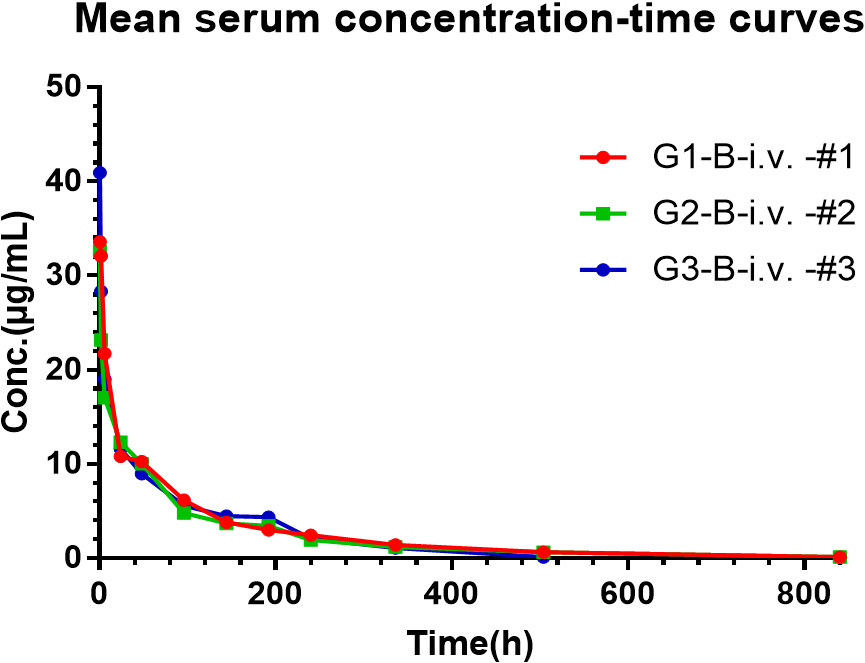
Fig. 2 serum concentration-time curves of BsAb B
Table 2 parameters curves of BsAb B
|
Group
|
Animal ID
|
T1/2
(h)
|
Cmax
(μg/ml)
|
Tmax
(h)
|
AUC 0-t
(h*μg/ml)
|
AUC 0-∞
(h*μg/ml)
|
CL
(ml/h/kg)
|
MRT0-∞
(h)
|
|
G1 B i.v.
|
1
|
148.07
|
33.57
|
0.50
|
2165.66
|
2194.60
|
2.06
|
161.45
|
|
2
|
160.43
|
32.58
|
0.50
|
2028.51
|
2060.49
|
2.19
|
167.71
|
|
|
3
|
150.83
|
40.90
|
0.50
|
2025.70
|
2053.78
|
2.01
|
128.81
|
|
|
mean
|
153.11
|
35.68
|
0.50
|
2073.29
|
2102.96
|
2.09
|
152.66
|
|
|
SD
|
6.49
|
4.54
|
0.00
|
80.01
|
79.44
|
0.10
|
20.89
|










